Published: 2026-01-22 01:00
EvoMDT: AI Multi-Agent System for Structured Multi-Cancer Clinical Decisions
The landscape of oncology is increasingly complex, driven by advances in diagnostics, an expanding array of therapeutic options, and a growing understanding of cancer heterogeneity. Clinicians routinely navigate vast amounts of patient data, from imaging and pathology to genomics and treatment history, often within the collaborative framework of multidisciplinary teams (MDTs). The challenge intensifies when patients present with multiple primary cancers, complex metastatic disease, or rare tumour types, demanding highly nuanced and integrated decision-making.
Into this intricate environment, a new paradigm in artificial intelligence (AI) is emerging: the self-evolving multi-agent system. One such innovation, dubbed EvoMDT, proposes a novel approach to structured clinical decision-making, particularly in the challenging realm of multi-cancer cases. Published in *npj Digital Medicine*, this system represents a significant step towards leveraging AI not just for data analysis, but for simulating and supporting the complex, iterative process of clinical reasoning that underpins MDT discussions.
Navigating Complexity: The MDT Imperative
Multidisciplinary team meetings are the cornerstone of cancer care in the UK, bringing together oncologists, surgeons, radiologists, pathologists, specialist nurses, and other allied health professionals. Their collective expertise aims to formulate optimal, personalised treatment plans based on a holistic view of the patient and their disease. However, the sheer volume of information, the rapid pace of medical advancements, and the inherent complexities of individual cases can strain even the most experienced MDTs.
For patients with multiple primary cancers, or those with highly aggressive or refractory disease requiring intricate sequential or combined therapies, the decision-making process becomes exponentially more challenging. Factors such as treatment sequencing, potential drug interactions, cumulative toxicities, and the patient’s overall fitness must all be meticulously weighed. This is where AI systems like EvoMDT aim to provide robust, evidence-based support, potentially enhancing the efficiency and consistency of MDT recommendations.
Understanding EvoMDT: A Collaborative AI Approach
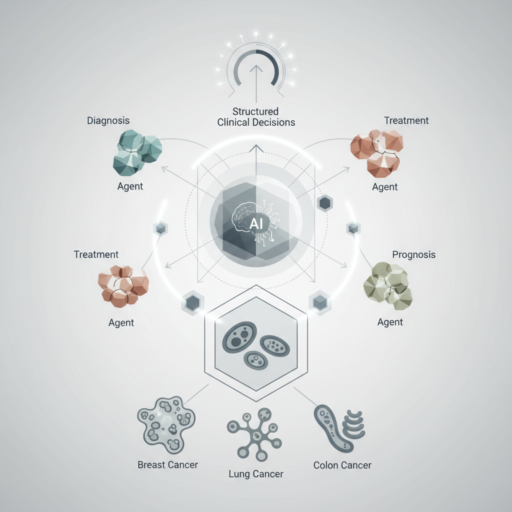
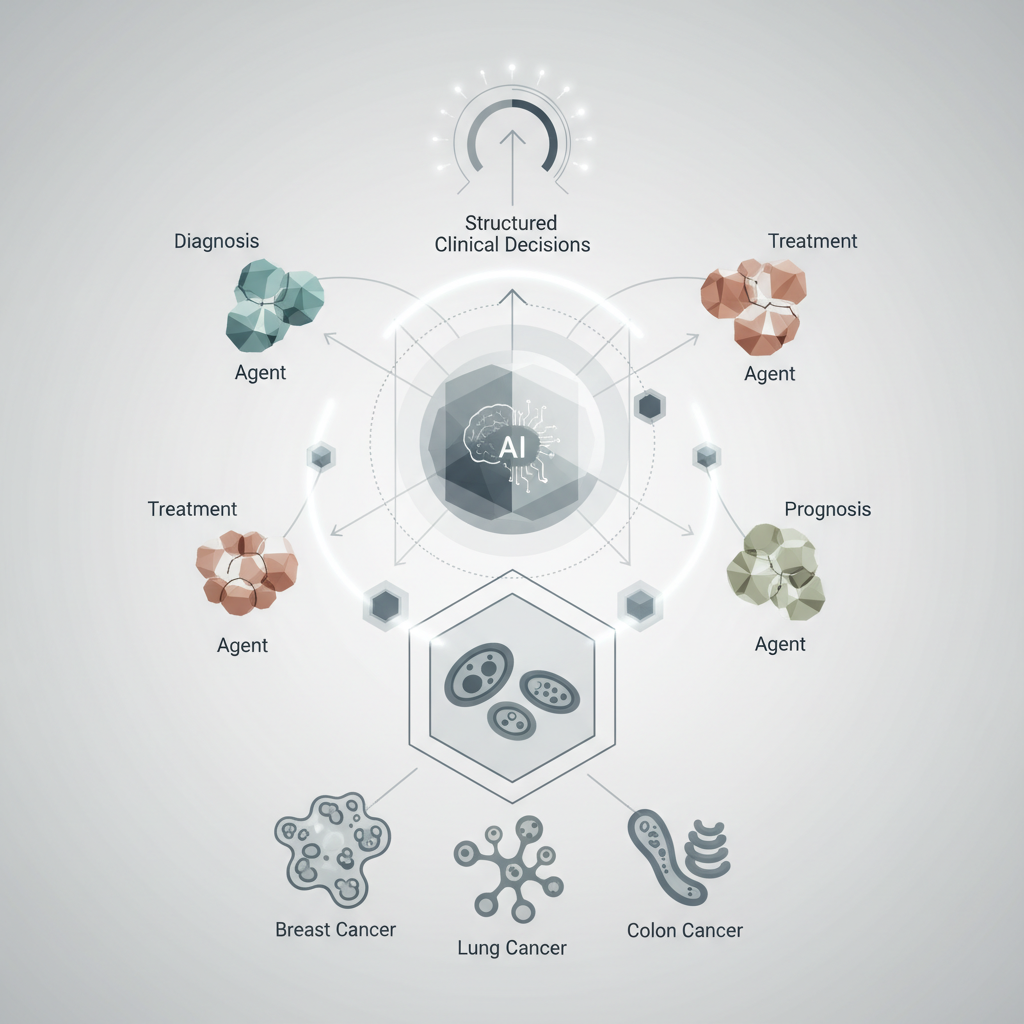
At its core, EvoMDT is described as a “self-evolving multi-agent system.” This terminology is crucial for understanding its potential capabilities and distinguishing it from more conventional AI models.
* Multi-Agent System: Unlike a single, monolithic AI algorithm designed for a specific task (e.g., image classification), a multi-agent system comprises several independent AI entities, or ‘agents,’ each with a specialised function. These agents interact, communicate, and collaborate to achieve a common goal – in this case, generating comprehensive clinical decision support. Imagine a digital MDT, where each ‘agent’ acts as a specialist, contributing its expertise to a shared problem.
* Self-Evolving: This characteristic implies that the system is not static. It is designed to learn and adapt over time, continuously refining its knowledge base and decision-making algorithms. This evolution can occur through various mechanisms, such as incorporating new clinical data, learning from patient outcomes, integrating updated guidelines, and even receiving feedback from human clinicians. This adaptive capacity is vital in oncology, where research and treatment paradigms are constantly advancing.
The combination of these two features suggests a system capable of dynamic, integrated reasoning, moving beyond simple pattern recognition to a more sophisticated form of clinical intelligence.
How EvoMDT Functions: A Glimpse into the Digital MDT
While specific architectural details would require deeper engagement with the research paper, the concept of EvoMDT implies a structured workflow that mirrors, and potentially augments, human clinical reasoning.
1. Data Ingestion and Integration: The system would likely begin by ingesting a vast array of patient data. This could include:
* Demographics and Medical History: Age, comorbidities, previous treatments, family history.
* Imaging Data: CT, MRI, PET scans – often requiring sophisticated image analysis agents.
* Pathology Reports: Histology, immunohistochemistry, tumour grading.
* Molecular and Genomic Data: Gene mutations, expression profiles, biomarkers.
* Treatment Guidelines: National and international protocols (e.g., NICE guidelines, ESMO, NCCN).
2. Agent Specialisation and Interaction: Once data is ingested, different AI agents would likely take on specific roles:
* Diagnostic Agent: Interpreting imaging and pathology to confirm diagnoses, stage disease, and identify primary vs. metastatic sites.
* Genomic Agent: Analysing molecular data to identify actionable mutations, predict treatment response, and assess prognosis.
* Treatment Planning Agent: Synthesising diagnostic and genomic information with clinical guidelines to propose potential therapeutic strategies (e.g., surgery, radiotherapy, systemic therapies like chemotherapy, targeted agents, immunotherapy).
* Risk Assessment Agent: Evaluating patient-specific factors (comorbidities, performance status) to predict treatment toxicities, surgical risks, and overall prognosis.
* Guideline Adherence Agent: Ensuring proposed plans align with current evidence-based guidelines and protocols, flagging any deviations for review.
* Outcome Prediction Agent: Using historical data to estimate the likelihood of various outcomes for different treatment paths.
3. Iterative Recommendation Generation: The agents would not work in isolation. They would communicate and negotiate, much like human specialists in an MDT. For instance, the Genomic Agent might identify a targetable mutation, which the Treatment Planning Agent then uses to suggest a specific therapy. The Risk Assessment Agent might then flag potential contraindications based on comorbidities, prompting the Treatment Planning Agent to explore alternative options or dose modifications. This iterative process would lead to a refined, comprehensive set of recommendations.
4. Structured Output and Justification: The final output from EvoMDT would not just be a recommendation, but a structured report detailing the rationale behind each suggestion, citing the evidence and data points considered. This transparency is crucial for clinician acceptance and for fulfilling regulatory requirements.
Addressing the Multi-Cancer Conundrum
One of the most compelling aspects of EvoMDT is its stated focus on “multi-cancer” decision-making. This is an area of significant unmet need. When a patient has two or more distinct primary cancers, or a complex metastatic pattern, the standard treatment pathways for individual cancers may conflict or require significant modification.
Consider a patient with early-stage breast cancer and a newly diagnosed, unrelated prostate cancer. The treatment for one might impact the other, or the patient’s overall tolerance for aggressive therapy might be limited. EvoMDT could potentially:
* Integrate Conflicting Guidelines: Synthesise recommendations from different cancer-specific guidelines, identifying potential synergies or conflicts.
* Optimise Treatment Sequencing: Suggest the most logical order of therapies to maximise efficacy while minimising cumulative toxicity.
* Personalise Risk-Benefit Analysis: Provide a more granular assessment of the overall risk-benefit profile for complex treatment regimens, considering all active malignancies and patient comorbidities simultaneously.
* Identify Novel Combinations: Potentially uncover less obvious but effective treatment combinations or sequences by analysing vast datasets of patient outcomes.
This holistic, integrated approach could lead to more coherent and effective treatment strategies for patients with complex multi-cancer presentations, a scenario that often presents significant challenges even for highly experienced MDTs.
The “Self-Evolving” Advantage: Keeping Pace with Progress

The self-evolving nature of EvoMDT is particularly pertinent in oncology, a field characterised by rapid innovation. New drugs, diagnostic techniques, and clinical trial results are published constantly, making it a formidable task for clinicians to stay abreast of all developments.
A self-evolving system could:
* Continuously Update Knowledge: Automatically ingest and process new research findings, clinical trial data, and updated guidelines, integrating them into its decision-making algorithms.
* Learn from Outcomes: By linking its recommendations to actual patient outcomes, the system could learn which strategies are most effective in specific patient cohorts, refining its predictive models over time. This feedback loop is critical for improving accuracy and relevance.
* Adapt to Local Practice: With appropriate training and validation, the system could potentially adapt to nuances in local clinical practice or resource availability, while still adhering to core evidence-based principles.
This continuous learning capability ensures that the AI remains relevant and cutting-edge, potentially offering insights derived from a knowledge base far exceeding what any individual clinician or even an MDT could realistically maintain.
Potential Benefits for UK Clinicians and the NHS
The introduction of a system like EvoMDT into the UK healthcare system could offer several tangible benefits:
* Enhanced Decision Support: Providing MDTs with a comprehensive, evidence-based second opinion, particularly for complex or rare cases, could bolster confidence and consistency in decision-making.
* Standardisation and Quality Improvement: By integrating national guidelines and best practices, EvoMDT could help standardise care pathways, reducing unwarranted variation in treatment across different trusts or regions.
* Increased Efficiency: Automating the synthesis of vast amounts of patient data and guideline information could free up valuable clinician time, allowing MDTs to focus more on nuanced discussions and patient communication rather than data collation.
* Personalised Medicine: By integrating genomic and other ‘omics’ data with clinical information, the system could facilitate truly personalised treatment plans tailored to the individual patient’s tumour biology and clinical profile.
* Training and Education: Junior clinicians could use the system’s structured rationale to understand complex decision-making processes, serving as an educational tool.
* Research Facilitation: The aggregated, anonymised data processed by such a system could also be a valuable resource for epidemiological studies, treatment outcome analysis, and identifying areas for future research.
It is crucial to frame EvoMDT not as a replacement for human expertise, but as a powerful augmentative tool. The ultimate decision-making authority and responsibility would always remain with the human clinicians, who bring invaluable empathy, ethical judgment, and an understanding of patient preferences that AI cannot replicate.
Challenges and Considerations for Implementation in the UK
While the promise of EvoMDT is significant, its successful integration into the NHS would necessitate addressing several critical challenges:
Data Governance and Security
The NHS holds highly sensitive patient data. Any AI system handling such information must adhere to stringent data protection regulations (e.g., GDPR, Data Protection Act 2018). This includes:
* Anonymisation and Pseudonymisation: Ensuring patient data is appropriately de-identified before being used for training or processing by the AI.
* Secure Data Handling: Robust cybersecurity measures to protect against breaches.
* Consent: Clear protocols for obtaining patient consent for their data to be used in AI systems.
* Interoperability: Seamless and secure integration with existing NHS IT infrastructure, electronic health records (EHRs), and diagnostic systems.
Regulatory Approval and Clinical Validation
As a medical device, EvoMDT would require rigorous regulatory approval from bodies like the Medicines and Healthcare products Regulatory Agency (MHRA). This would involve:
* Demonstrating Safety and Efficacy: Extensive clinical validation studies to prove that the system’s recommendations are accurate, safe, and lead to improved patient outcomes.
* Transparency and Explainability: The ‘black box’ problem of AI must be addressed. Clinicians need to understand *how* the AI arrived at its recommendations to trust and verify them.
* Bias Detection: Rigorous testing to ensure the AI does not perpetuate or amplify biases present in its training data, which could lead to health inequalities.
Ethical Considerations and Accountability
The ethical implications of AI in clinical decision-making are profound:
* Accountability: In the event of an adverse outcome, who is accountable? The AI developer, the clinician who followed the recommendation, or the institution? Clear frameworks are needed.
* Autonomy: Ensuring that AI recommendations do not undermine clinician autonomy or patient choice.
* Equity: Preventing AI from exacerbating existing health inequalities, for example, if its training data is not representative of diverse patient populations.
Clinician Acceptance and Training
For any new technology to succeed, clinicians must trust it and be willing to adopt it. This requires:
* Effective Training: Comprehensive training programmes for MDT members on how to use EvoMDT, interpret its outputs, and integrate it into their workflow.
* Building Trust: Demonstrating the system’s reliability and value through transparent validation and ongoing performance monitoring.
* Addressing Concerns: Openly addressing clinician concerns about job displacement, over-reliance on AI, or loss of clinical judgment.
Resource Implications
Implementing and maintaining such a sophisticated AI system will require significant investment in:
* Infrastructure: High-performance computing, secure data storage.
* Expertise: Data scientists, AI engineers, clinical informaticists to manage and optimise the system.
* Ongoing Development: Continuous updates, maintenance, and refinement of the AI.
The Future of AI in Oncology: A Collaborative Vision
EvoMDT represents a compelling vision for the future of AI in oncology – one where intelligent systems act as collaborative partners, rather than mere tools. As AI technologies mature, their role in precision medicine is set to expand dramatically.
Beyond decision support, AI could contribute to:
* Drug Discovery and Development: Accelerating the identification of new therapeutic targets and compounds.
* Clinical Trial Design: Optimising patient selection and trial protocols.
* Early Detection and Screening: Improving the accuracy and efficiency of cancer screening programmes.
* Prognostic Modelling: Providing more accurate individualised prognoses.
The journey from a promising research concept like EvoMDT to widespread clinical adoption is long and complex.
Source: Nature